Saving living diversity in the face of the
unstoppable 6th mass extinction: A call for urgent international action
Fred
Naggs
Fred
Naggs is a Scientific Associate at the Natural History Museum, having retired
after 42 years at the Museum in September 2016. Initially the Curator of
non-marine Mollusca, Fred was appointed as the Biodiversity & Conservation
Officer in 2003. He established international collaboration and ran programmes
throughout south and much of tropical south-east Asia. He is a visiting
professor at Chulalongkorn University, Bangkok.
freddynaggs@gmail.com
–––––––––––––––––––––––––––––––––––––––––––
DOI: 10.3197/jps.2017.1.2.67
Licensing: This article is Open Access (CC BY 4.0).
How to Cite:
Naggs, F. 2016. 'Saving living diversity in the face of the unstoppable 6th mass extinction: A call for urgent international action'. The Journal of Population and Sustainability 1(2): 67–81.
https://doi.org/10.3197/jps.2017.1.2.67
–––––––––––––––––––––––––––––––––––––––––––
Abstract
The global scale and impact of
current and increasing human population size is incompatible with the survival
of biological diversity and the 6thmass extinction cannot be stopped. For the vast majority
of species we have neither the knowledge of when they will go extinct nor the
capacity to find out. Conventional conservation measures can only amount to
token damage limitation. Advances in molecular biology allow low cost options
for storing the genetic diversity of numerous species and maximising future
options for restoring species.
Keywords: mass extinction; conservation;
cryobanking; land snails; international collaboration.
Introduction
We
are witnessing a biodiversity crisis: the loss of a large proportion of living
diversity resulting from a wide range of events that can all be ultimately
attributed to human population growth and human activity. Nothing else is
involved. The scale and speed of extinction is widely thought to be
unprecedented since the mass extinction event that occurred 65 million years
ago, which marked the transition from the Mesozoic to the Cainozoic era of
geological time (Ceballos et al. 2010; Barnosky et al. 2011; Laurance et al.,
2014; Ceballos et al., 2015). Clearly there is an urgent need to enact
conservation measures that will seek to safeguard remaining natural habitats,
to evaluate the relative conservation value of transformed habitats and to
restore habitat connectivity. However, barring a catastrophic human population
reduction, the process of massive extinctions cannot be stopped and can only be
moderated to a very limited extent (Naggs and Raheem, 2014). We are shielded
from this stark reality by a lack of honesty and willingness to admit
collective human culpability. It is not possible to control this situation in
the short or medium term and we cannot know how this calamity will play out,
other than the fact that there is no happy ending in prospect. The long-term
hope must be that at some point in the future mankind will exist in reduced
numbers with improved stewardship that will allow a sustainable existence in
relative harmony with the natural world. The problem with this scenario is that
unless we act with urgency and purpose, there won’t be much of a natural world
left to live in harmony with.
Perhaps
more shocking than widespread apathy is that organisations entrusted with
responsibility for recording and understanding biodiversity offer only the pretence
of responding to the biodiversity crisis. Almost without exception,
international museums with the remit of recording and understanding the natural
world exhibit a lamentable failure to address the biodiversity crisis let alone
act in a relevant way, although they have the capacity to do so. The
international Convention on Biodiversity (CBD) has largely been diverted to a
completely different agenda, epitomised by Britain’s Darwin Initiative’s shift
to poverty alleviation as a core objective, driven in part by the way in which
government funding is channelled (Darwin Initiative Secretariat 2014).
This seemingly worthy objective might appear to be beyond criticism but it
represents a hijacking of its supposedly biodiversity conservation intent. A
CBD target to halt extinctions by 2020 (Hochkirch, 2016) is out of touch with
reality. There is a prevailing lack of honesty about the extent to which, by
the scale of our existence, human utilisation of the planet to satisfy human
needs and voracity is driving extinctions, and about our inability to control
the process. While conservation as a scientific discipline has flourished, it
has failed to halt the process of massive habitat loss and consequent
extinctions (Whitten, et al., 2001; Wunder, 2001). Having witnessed the ongoing
and appalling scale of rainforest loss and degradation in large areas of south-east
Asia over the past few decades, I am under no illusion as to the magnitude and
reality of the biodiversity crisis.
Although
human driven species extinction often gains media coverage and arouses episodes
of anguish, attention is almost invariably drawn to an iconic vertebrate and
usually a mammal species. Just occasionally an invertebrate makes the
headlines. It is often overlooked that over 99% of multicellular animals are
invertebrates (Lunney and Ponder, 1999), many of which have disappeared and continue
to disappear without our having known of their existence (Lydeard et al. 2004;
Régnier et al. 2015; Hochkirch, 2016). Here I draw on my knowledge of land
snails, a major invertebrate group, to illustrate some key issues in the
biodiversity crisis. Snails serve to demonstrate that although extinctions are
happening on a massive scale it is more or less a waste of time to attempt to
critically evaluate ever more data on current extinctions. The ultimate scale
of the current mass extinction will be recognised long after the damage is done
but we will have little idea of what has been lost. Research is absolutely
necessary for furthering our understanding of the natural world but the broad
picture on extinction is clear; we need to focus our efforts on delivering
solutions.
More
in the realm of science fiction than reality, when faced with mortality, some
human beings seek a solution by having their bodies cryogenically preserved in
the hope that they can be woken in a future, advanced world, where they can be
restored to life. However, the cryogenic storage of viable cells of living
organisms has moved beyond the realm of science fiction. Advances in molecular
biology allow us to store the genetic diversity of species and potentially
restore species should they become extinct (Lerman et al., 2009). This is the
new reality that allows us the only route for storing living diversity on a
scale that is commensurate with its current levels of loss. It offers a
long-term strategy that extends way beyond a human lifetime but as a course of
action it is entirely doable and fundable, if the will to do so can be
summoned. This is about maximising future options. We may not be able to
preserve all living diversity, but we can aim to do so and the sooner we act
the greater the chance of preserving as many species as possible before they
disappear.
Islands and disappearing snails
Oceanic
islands have a special significance for evolutionary biologists as natural
laboratories that model events in the wider world. Islands also represent the
delicate canary in the coal mine of the world’s natural environments. As an
outstanding observer of the natural world and armed with a copy of Charles
Lyell’s newly published Principles of Geology,
Charles Darwin was well equipped to read landscapes and interpret their
history. From the first landing on the voyage of the Beagle at the Cape Verde
Islands, Darwin had immediately recognised the impact of human activity on
natural habitats. “When the island was discovered, the immediate neighbourhood
of Porto Praya was clothed with trees, the reckless destruction of which has
caused here, as at St. Helena, and at some of the Canary islands, almost entire
sterility” (1845 [1839], p 2). On 8th July 1836, towards the end of Darwin’s
voyage on the Beagle, a brief stop of a few days was made at the isolated
island of St Helena in the Atlantic Ocean. With his notable powers of
perception Darwin recognised that the island had been transformed by human
occupation and that this had led to the loss of its native forest and numerous
species of invertebrates:
On
the higher parts of the island, considerable numbers of a shell, long thought
to be a marine species, occur embedded in the soil. It proves to be a
Cochlogena, or land-shell of a very peculiar form; with it I found six other
kinds; and in another spot an eighth species. It is remarkable that none of
them are now found living. Their extinction has probably been caused by the
entire destruction of the woods, and the consequent loss of food and shelter,
which occurred during the early part of the last century…. There can be little
doubt that this great change in the vegetation affected not only the
land-shells, causing eight species to become extinct, but likewise a multitude
of insects. (Ibid. pp 469 – 471) (figure 1).

Figure 1: Chilonopsis nonpareil (Perry, 1811) [Chilonopsis = Cochlogena sensu Darwin].
There can be little doubt that this medium sized snail had been extinct for
many years prior to Darwin’s observations of subfossil shells on St Helena in
1836. Nevertheless, some of the shells look as fresh as those of a living snail
and they are found with what are likely to be their eggs. Using a shell and
preserved eggs this image reconstruction shows what a living example might have
looked like. Image prepared by Harold Taylor ©Natural History Museum.
Recorded
extinctions of land snails are disproportionately high and there is clear
evidence for snail extinctions over the past few hundred years that exceed
recorded extinctions for all other animal groups combined. From the Hawaiian
Islands alone Cowie et al., (1995) estimated that some 570 of the 763 species
listed in their catalogue are probably extinct and this does not take account
of the approximately 200 species of ‘known’ but undescribed species of now
extinct Hawaiian charopid snails in the Bishop Museum (Naggs et al., 2006).
Compare this with the total of 484 human induced extinctions cited by
Groombridge (1992) for all animal groups, which includes a mere 191 molluscs.
The IUCN Red List (2015) includes 832 species listed as extinct since 1600 and
there are regular calls for more research into establishing detailed
information for current extinction levels (Hayward, 2009). Although it is widely
recognised that the level of evidence and how it is interpreted vary enormously
(Regan et al., 2005), efforts continue to be made to refine and justify hard
data. Several commendable and critical studies have attempted to establish
reliable, evidence-based assessments of land snail extinctions (Lydeard et al.,
2004; Régnier et al., 2009, Régnier et al., 2015). They come up with alarming
figures but we should be mindful of Darwin’s observation that extinctions of
land snails are a visible example of a multitude of other extinctions that do
not leave shells as a record of their passing. The 394 insect species recorded
as being extinct by the IUCN Red List of Threatened Species bears absolutely no
relation to reality (Hochkirch, 2016) and is meaningless. It might seem
reasonable to ask, as these researchers do, what detailed evidence is available
for current extinction levels. But is this missing the point? Firm figures are
often cited but I contend that very few invertebrate risk status evaluations
survive close inspection; we simply do not know. A more promising approach is
to estimate species loss by extrapolating from known habitat loss, but such
model-based studies (e.g. Beck, 2011) do not take us beyond the self-evident
reality that such approaches can only provide broad approximations. If we have
little idea of how many species there are (Caley et al., 2014; Giller, 2014),
how can we begin to know the rate of extinction? Because of massive habitat
loss and degradation, we can confidently infer that extinctions are happening
on a massive scale but geographical species turnover varies enormously from one
area to another, often for no discernible reason, and there is no simple way of
linking extinction to habitat loss. When it comes to specifics relating to small
animals and invertebrates in particular we are profoundly ignorant. Anyone who
is familiar with large reference collections of invertebrates will be aware
that many species have not been recorded again since they were first described.
Attempts, such as the IUCN Red List system applied to invertebrates are well
meaning but illusory. The transition from critically endangered to extinct is
indeed a profound and currently irretrievable step and we want to know about
when it happens but it is important not to compromise our credibility with
unwarranted certainty of the particular when it is the general picture that is
of paramount importance. To establish the status of a single invertebrate
species could take years of research and still be wrong. Asserting that a
particular tiny snail has just become extinct simply exposes researchers to
ridicule if just one example should later be found surviving in a remote
valley.
Unintended consequences: a
global pest, transmitter of human pathogens, wave of extinctions and a vision
for saving biodiversity
In
1847 William Benson, a civilian administrator in the service of the East India
Company and pioneer in the study of land snails in India (Naggs, 1997), brought
two Giant African Snails back with him from Mauritius to India. Released in a
Chowringhee garden after Benson left India, the snails slowly spread across
Calcutta (Benson, 1858; Blanford, 1868; Godwin-Austen, 1908) and have since
been recorded in every continent except for Antarctica. The species has since
become a serious agricultural pest and vector for a sometimes fatal disease in
humans (Alicata, 1966). Vast sums of money are spent on its control and local
eradication but its large size, extended distribution range and the high
densities populations often reach render it likely to have the highest biomass
of any species of snail (Budha and Naggs, 2005). Following their introduction
to a new area, L. fulica often reach plague proportions and this
is what happened when they were released in Tahiti in 1967. They soon spread
throughout the archipelago, including the island of Moorea.
An
ill-conceived but widely advocated biological control method for L. fulica, based on setting a snail to catch a
snail, was initiated with the release of several species of predatory snail in
areas where L. fulica had become established. The most
‘successful’ of these introductions was of the voracious predatory species Euglandina rosea. There was no evidence that E. rosea would
be an effective control agent of L. fulica and it proved not to be but it was very
successful in killing local endemic species. E. rosea has
caused devastation to the endemic land snail faunas on Indian Ocean and Pacific
Ocean islands. Early and thorough evidence of this came to the notice of the
scientific community (Tillier and Clarke, 1983; Clarke et al. 1984) because the
land snails of Moorea, their abundance and distribution, were known in great
detail, most notably the endemic genus Partula. The genetics and distribution of Partula had
been studied for decades as a model system for investigating speciation and
evolution (Crampton, 1932; Murray and Clarke, 1980; Murray et al., 1982).
Bryan
Clarke was a pioneer of ecological genetics and a central figure in the study
of Partula (Jones, 2014). After years of studying Partula Bryan
was deeply shocked to find that Partula species were rapidly becoming extinct and
this personal experience of extinction drove him to seek, if not a solution, a
strategy for addressing the issue of extinction. Bryan was instrumental in
setting up an international project for the captive breeding of Partula that
is coordinated by Paul Pearce Kelly at the Zoological Society of London[1]. Captive breeding of Partula was
successfully established and Partula species that became extinct in the wild
remain in captive breeding projects with a long-term aim to return them to
their natural homes[2]. The Partula story
is a flagship example of how zoos can perform an important role in conserving
species on the brink of extinction but something far more ambitious was needed
to be of any relevance to the scale of extinctions and this led to Bryan, his
wife Ann and Anne McLaren setting up the Frozen Ark in 1996[3].
The
initial objectives of the Frozen Ark were to establish repositories of frozen
tissue of endangered animals and to at least have a genetic record of animals
that might become extinct. The idea was to set up a global consortium of
partners in this venture, which currently includes 22 zoos and other research
institutions in eight countries. Bryan and Ann soon realised that the prospect
of restoring species from viable cells had moved from the realms of science
fiction to scientific reality and rather than simply store DNA the cryogenic
storage of viable cells became a Frozen Ark objective. The value of biobanking
or cryobanking as a conservation tool is recognised in some academic circles
(Lerman et al., 2009) but to date the only serious development of cryogenic
storage of species viable cells is undertaken at the San Diego Zoo, Institute
for Conservation Research, Frozen Zoo project[4]. However, valuable as these initiatives
are they do not yet begin to approach the scale that is needed.
Priorities for action and how
they can be delivered
Either
new institutions are required or existing institutions need to respond to the
challenge of establishing a worldwide programme to undertake surveys and store
viable cells of the whole range of living diversity. The institutional
requirements can be identified as:
1. Secure
long-term funding.
2. Teams
of appropriate scientific personnel.
3. Expertise
in data management
4. Capacity
to store cryogenic and conventionally preserved biological collections.
5. The
capacity to undertake large-scale collection based surveys.
6. An
institutionally shared vision and commitment to utilise these skills and
resources to build cryogenic collections as a means of species conservation.
Hochkirch
(2016) advocates the establishment of new institutions for invertebrate
conservation but, apart from item 6, it would seem that the world’s major
international museums that encompass life sciences meet all of these criteria.
However, in lacking both leadership on this issue and a relevant culture it may
be that these institutions cannot respond to the challenge[5],[6],[7].
Across the world only the Muséum national d’Histoire naturelle (MNHN), Paris,
is a major museum that has an ambitious collections programme attempting to
make twenty-first century collections to record living diversity, rather than
being preoccupied with historical collections that date mostly from the
nineteenth century. The MNHN programme[8] is
entirely due to the vision, energy and drive of one man, Philippe Bouchet. In
2009 Philippe Bouchet with colleagues at the MNHN and the NGO Pro-Natura
International launched ambitious plans to amass enormous collections of
reference specimens. They focussed on rich but poorly-known biotas under the
programme ‘La Planète Revisitée’ (‘Planet Reviewed’) a vast program of surveys
planned over 10 years. This massive undertaking is the most praiseworthy of any
of the world’s collection-based research institutions’ initiatives. It has
demonstrated that large-scale collecting is still achievable in a
bio-politicised world and that traditional morphological collections can be
integrated with DNA collections and molecular bar coding on a large scale
(Puillandre, et al., 2012). Hopefully, viable cell preparations and storage
will be added to their collection protocols. However, commendable as the Planet
Reviewed programme is, it is almost entirely directed at marine surveys. The
criteria for identifying priorities include areas of highest diversity,
endemism and threat. So far, the current wave of extinctions has occurred
almost entirely in non-marine environments. This has been most visible on
oceanic islands but is occurring largely unrecorded on continental land masses,
most notably in tropical rainforests.
Undertaking
large-scale collection surveys in tropical forests is far less expensive than
the major expenditure involved with marine surveys but it can be more
problematic. Until the mid-twentieth century it was possible to collect
specimens throughout much of the world with few restrictions. Collecting of
invertebrates in particular was perceived as an obscure obsession pursued by a
few eccentrics that very few cared about. However, in the past seventy years or
so there has been an ever-growing reluctance to allow specimen collections to
be made, particularly by non-nationals, and international collecting has become
increasingly difficult. The 1992 international Convention on Biological
Diversity (CBD) pushed biodiversity higher up the political agenda of nations
and reaffirmed that states have sovereign rights over their biological
resources. Whatever the intent of the CBD, the reality has been biodiversity
nationalism and barriers to international collaboration. Having run
collection-based survey programmes throughout south and much of tropical
southeast Asia over the past two decades I have been privileged to work with
international colleagues in various productive ways but our collaborations have
been significantly hampered by such constraints.
The
first step in establishing optimal collaboration is to set up international
agreements based on a common vision and shared objectives. Following five years
of collaborative projects with colleagues in Vietnam, I established an
agreement with the Vietnamese National Museum of Nature (VNMN) in 2010 that
allows international partners to work effectively. The key element is to share
survey material and allow for duplicate centres for the cryogenic preservation
of viable cells in addition to conventional voucher specimens and frozen tissue
samples. Supported by the Natural History Museum, the Zoological Society of
London and the Frozen Ark, I led a team with UK and Vietnamese colleagues in
2013 with the objective of undertaking a survey project that included viable
cell preparations. We successfully carried out a traditional survey, sampling
and preserving morphological voucher specimens and, in addition to preserving
tissue samples for molecular research, followed with the additional stage of
making viable cell preparations. This proved to be relatively straightforward
and was a procedure easily incorporated into our existing methodology for
processing specimens and transferring frozen samples to cryogenic storage
facilities at the Natural History Museum, London. Clearly such surveys need to
be integrated with research that refines and assesses the viability of cell
preparations. In many instances, it may prove possible to preserve viable
zygotes or gametes, obviating the need for cloning to restore species.
The
programme in Vietnam serves as a model system, demonstrating that large scale
biotic surveys and routine preparation of viable cells in the field are
relatively straightforward. The VNMN is committed to the process: it is
establishing a new museum with ambitious biobanking facilities, embarking on
nationwide biotic surveys and actively pursuing collaborations with
international partners. Similar schemes urgently need to be taken up by the
international scientific community and government agencies. It is our best hope
for maximising options for restoring a biodiverse world (figure 2).
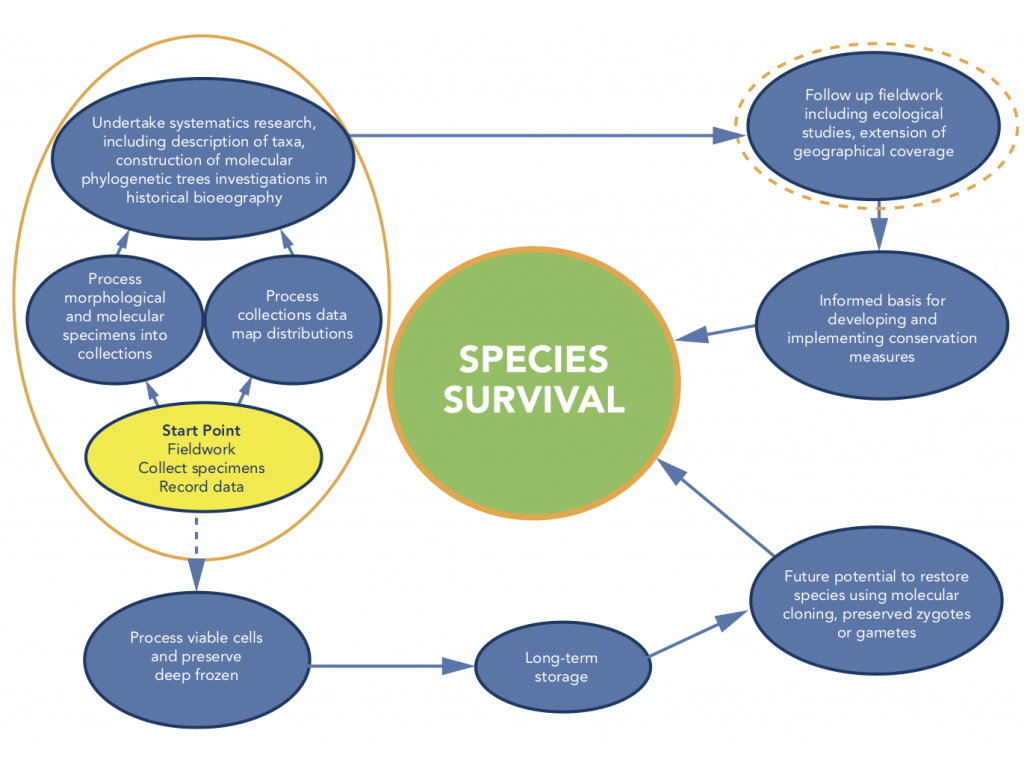
Figure 2: International
museums’ historical specimen collections are priceless and provide the
foundation for naming the world’s biota. However, they do not begin to meet the
needs of current and future research, which requires state of the art
collection methods of preservation, georeferenced localities and habitat data.
The rate of extinctions imposes an urgent need for recording the current extent
of living diversity and establishing a global inventory. It is a small
additional step to include the preservation of viable cells. In addition to
providing optimal material for research, preservation of viable cells provides
a mechanism for safeguarding genetic diversity and allows for the possibility
of restoring species should they become extinct. This offers a long-term option
and alternative route for conserving living diversity that complements
traditional conservation measures.
Calls
for prioritising the description of new species are misplaced in the context of
viable cell conservation and the assertion that we can only preserve what we
know (Hochkirch, 2016) does not apply to broad-based survey collections: you
collect what you find. Obviously, it is desirable to name new species as soon
as possible and molecular tools can facilitate this but if viable cells and
morphological voucher specimens are preserved, we have a very long time at our
disposal to describe them. If they are gone, we have nothing.
[1] See https://www.zsl.org/conservation/regions/oceania/partula-snail-conservation-programme
[2] See https://www.zsl.org/zsl-london-zoo/news/release-the-snails
[3] See https://frozenark.org/
[4] See http://institute.sandiegozoo.org/resources/frozen-zoo%C2%AE
[5] https://www.si.edu/Museums
[6] http://www.nhm.ac.uk/about-us/our-vision-strategy.html
[7] http://www.nhm.ac.uk/research-curation/about-science/science-directorate/science-strategy/
[8] See https://www.mnhn.fr/en/research-expertise/scientific-expeditions/our-planet-reviewed
References
Abbott,
R., 1989. Compendium of landshells.
Melbourne, Florida: American Malacologists.
Alicata,
J., 1966. The presence of Angiostrongylus Cantonensis in the islands of the Indian Ocean and
probable role of the giant African snail, Achatina Fulica, in the Dispersal of the
parasite to the Pacific Islands. Canadian Journal of Zoology, 44
pp. 1041–1049.
Barnosky,
A.D., Matzke, N. Tomiya, S., Wogan, G.O.U. Swartz, B., Quental, B., Marshall,
C. McGuire, J.L., Lindsey, E.L., Maguire, K.C., Mersey, B. and Ferrer, E.A.,
2011. Has the Earth’s sixth mass extinction already arrived? Nature, 471 pp. 51–57.
Beck,
J., 2011. Species–area curves and the estimation of extinction rates. Frontiers of Biogeography 3(3) pp. 81-82.
Benson,
W.H., 1858. Note sur la transportation et al naturalisation au Bengale de l’achatina fulica de
Lamarck. Jouranal de Conchyliologie, 1, pp. 266-268.
Blanford,
W.T., 1868. On the occurrence of Diplommatina huttoni and Ennea bicolor in the
West Indies. Annals and Magazine of Natural
History, 4 pp. 110–112.
Budha,
P.B., Naggs, F., 2005. The giant African land snail Lissachatina fulica(Bowdich) in Nepal. The Malacologist, 45 pp. 19-21.
Caley,
M.J., Fisher, R., Mengersen, K., 2014. Global species richness estimates have
not converged. Trends in Ecology &
Evolution, 29(4) pp. 187–188.
Ceballos,
G., García, A., and Ehrlich, P.R., 2010. The sixth extinction crisis: Loss of
animal populations and species. Journal of Cosmology, 8
pp. 1821-1831.
Ceballos,
G., Ehrlich, P.R., Barnosky, A.D., García, A., Pringle, R.M., and Palmer, T.M.,
2015. Accelerated modern human–induced species losses: Entering the sixth mass
extinction. Science Advances, 19 Jun 2015, 1(5): e1400253
Clarke,
B., Murray, J. and Johnson, M. S. 1984. The extinction of endemic species by a
program of biological control. Pacific Science, 38(2), pp. 97-104.
Cowie,
R.H., Evenhuis, N.L., and Christensen, C.C., 1995. Catalog of the native land and freshwater mollusks of the Hawaiian
Islands, Leiden: Backhuys.
Crampton,
H . E. 1932. Studies on the variation, distribution, and evolution of the genus
Partula. The species inhabiting Moorea. Carnegie Institute of Washington,
Publication 410, pp. 1-335.
Darwin,
C. 1845. Journal of researches into the
natural history and geology of the countries visited during the voyage of
H.M.S. Beagle round the world. Second Edition (original
published in 1839) London: Murray.
Darwin
Initiative Secretariat, 2014. Learning note: poverty and the
Darwin Initiative. [pdf] LTS International. Available at: <http://www.darwininitiative.org.uk/assets/uploads/2014/05/DI-Learning-Note-poverty-and-biodiversity-2014-Final.pdf>
[Accessed: 21 December 2016]
Giller,
G., 2014. Are we any closer to knowing how many species there are on earth? Are
there half a million? 100 Million? After decades of research, there is no
consensus. Scientific American April 8, 2014. Available at: https://www.scientificamerican.com/article/are-we-any-closer-to-knowing-how-many-species-there-are-on-earth/ [Accessed: 21 December 2016]
Godwin-Austen,
H.H., 1908. The dispersal of land shells by the agency of man. Proceedings of the Malacological Society of London, 8
pp. 146–147.
Groombridge,
B., (Ed.), 1992. Global biodiversity: status of
the earth’s living resources : a report compiled by the World Conservation
Monitoring Centre in collaboration with the Natural History Museum, London; and
in association with IUCN-the World Conservation Union.
London; New York: Chapman & Hall.
Hayward,
M. W., 2009. The need to rationalize and prioritize threatening processes used
to determine threat status in the IUCN Red List. Conservation Biology, 23, pp. 1568–1576.
Hildyard,
A (Ed.), 2001. Endangered wildlife and plants of the world. International Wildlife Encyclopedia, 10
pp. 1299-1440.
Hochkirch,
A., 2016. The insect crisis we can’t ignore. Nature, 539 p. 141.
IUCN
Red List, 2015. Available at: http://www.iucnredlist.org/ [Accessed: 21 December 2016]
Jones,
S., 2014. Obituary Bryan Clarke. Heredity, 112
pp. 569–570.
Laurance,
W.F., Sayer, J., and Cassman, K.G., 2014. Agricultural expansion and its
impacts on tropical nature. Trends in Ecology and Evolution, 29
pp. 107-116.
Lerman,
D., Blömeke, B., Browne, R., Clarke, A., Dyce, P.W., Fixemer, T. B., Fuhr,
G.R., Holt, W.V., Jewgennow, K., Lloyd, R. E., Lötters, S., Paulus, M.,
McGregor Reid, G., Rapopor, D.H., Rawson, D., Ringleb, J. Ryder, O.A., Spörl,
G.I., Schmitt, T., Veith, M., and Müller, P., 2009. Cryobanking of viable
biomaterials: implementation of new strategies for conservation purposes. Molecular Ecology, 18
pp. 1030–1033
Lydeard,
C., Cowie, R.H., Ponder, W.F., Bogan, A.E., Bouchet, P., Clark, S.A., Cummings,
K.S., Frest, T.J., Gargominy, O., Herbert, D.G., Hershler, R., Perez, K.E.,
Roth, B., Seddon, M., Strong, E.E., and Thompson, F.G., 2004. The global
decline of nonmarine mollusks. BioScience 54 (4) pp. 321-330.
Lunney,
D., and Ponder, W. F., (Eds.), 1999. The other 99%: the conservation
and biodiversity of invertebrates. Mosman: Royal Zoological
Society of New South Wales.
Murray,
J. and Clarke, B. 1980. The genus Partula on Moorea: Speciation in progress.
Proceedings of the Royal Society London Series B, 211, pp. 83-117.
Murray,
J., Johnson, M. S., and Clarke, B. 1982. Microhabitat differences among
genetically similar species of Partula. Evolution, 36, pp. 316-325.
Naggs,
F., 1997. William Benson and the early study of land snails in British India
and Ceylon. Archives of Natural History,
24(1) pp. 37-88.
Naggs,
F., Panha, S., and Raheem, D., 2006. Developing land snail expertise in south
and southeast Asia, a New Darwin Initiative Project. The Natural History Journal of Chulalongkorn University, 6(1) pp.43-46.
Naggs,
F. and Raheem, D. 2014. Preface. In: Raheem, D.C., Taylor, H., Ablett, J.,
Preece, R.C., Aravind, N.A., and Naggs, F. A systematic revision of the land
snails of the Western Ghats of India. Tropical Natural History, Supplement 4 pp. 1-294.
Puillandre,
N., Bouchet, P., Boisselier-Dubayle, M.C., Brisset, J., Buge, B., Castelin, M.,
Chagnoux, S., Christophe, T., Corbari, L., Lambourdière, J., Lozouet, P.,
Marani, G., Rivasseau A., Silva, N., Terryn, Y., Tillier, S., Utge, J., Samadi,
S., 2012. New taxonomy and old collections: integrating DNA barcoding into the
collection curation process. Molecular Ecology Resources 12 pp 396-402.
Regan,
T. J., Burgman, M.A., McCarthy, M.A., Master, L.L., Keith, D.A., Mace, G.M.,
and Andekman, S.J., 2005. The Consistency of extinction risk classification
protocols. Conservation Biology, 19
pp. 1969–1977.
Régnier,
C., Fontaine, B., and Bouchet, P., 2009. Not knowing, not recording, not
listing: numerous unnoticed mollusk extinctions. Conservation Biology, 23 pp. 1214–1221.
Régnier,
C., Achaz, G., Lambert, A., Cowie, R.H., Bouchet, P., and Fontain, B., 2015.
Mass extinction in poorly known taxa. Proceedings of the National
Academy of Sciences, 112 (25) pp. 7761–7766.
Tillier,
S., and Clarke, B.C., 1983. Lutte biologique et destruction du patrimoine
génétique: le cas des mollusques gastéropodes pulmonés dans les territoires
français du Pacifique. Génétique, Sélection,
Evolution, 15(4)
pp. 559-566
Whitten,
T., Holmes, D., and Mackinnon, K., 2001. Conservation biology: a displacement
behaviour for academia. Conservation Biology,
15(1) pp. 1-3.
Wunder,
S. 2001. Poverty alleviation and tropical forests—what scope for synergies? World Development, 29 pp. 1817–1833.