A tale of two islands. The reality of
large-scale extinction in the early stages of the Anthropocene: a lack of
awareness and appropriate action.
First online: 3 December 2019
Fred
Naggs
Fred
Naggs is a Scientific Associate at the Natural History Museum, having retired
after 42 years at the Museum in 2016. Initially the Curator of non-marine
Mollusca, Fred was appointed as the Biodiversity & Conservation Officer in
2003. He established international collaboration and ran programmes throughout
south and much of tropical south-east Asia. He is a visiting professor at
Chulalongkorn University, Bangkok.
freddynaggs@gmail.com
–––––––––––––––––––––––––––––––––––––––––––
DOI: 10.3197/jps.2019.4.1.15
Licensing: This article is Open Access (CC BY 4.0).
How to Cite:
Naggs, F. 2016. 'A tale of two islands. The reality of large-scale extinction in the early stages of the Anthropocene: a lack of awareness and appropriate action'. The Journal of Population and Sustainability 4(1): 15–42.
https://doi.org/10.3197/jps.2019.4.1.15
–––––––––––––––––––––––––––––––––––––––––––
Abstract
The
endemic biotas of oceanic islands were vulnerable and many have been lost. The
more ancient, complex and dynamic biotas of continents were more resilient but
are now being obliterated. Sumatra and Madagascar are large continental plate
islands with very different histories and biotas that exemplify the situation
on continental land masses. Both tropical islands have suffered massive habitat
loss and species extinction from human population pressure, Sumatra mostly from
global and Madagascar from local pressure. Snails demonstrate the complex
history of faunal origins as illustrated by the relationships between
Madagascan, Indian and southeast Asian snail faunas and their plate tectonic
geological history. Snails also reveal our limited knowledge of the details but
not the scope of extinctions through habitat loss. International agencies are
failing to address the root causes of natural habitat loss and consequent
extinctions, which are overpopulation and an economic system based on perpetual
growth. The fallacy of sustainable development and the limitations of current
conservation practice are addressed. Recognition that we cannot stop
extinctions in the immediate future demands a new, supplementary approach to
conservation based on advances in molecular technology.
Keywords:
Sumatra; Madagascar; conservation; sustainable development; land snails;
cryo-banking.
Introduction
From
a negligible figure just a few thousand years ago humans and their livestock
now constitute over 95% of mammalian biomass (Bar-On et al., 2018). From an
ecological perspective, there are simply too many of us. The biodiverse world
that we were born into is disappearing and many branches of life will not be
with us in the future. Much attention is focussed on the threats to a few iconic
species but the extent of extinctions remains largely hidden, unknown in detail
but indisputable in scale. We need to be aware of what we are losing. The
earliest undisputed evidence of life on Earth dates from at least 3.5 billion
years ago and there is evidence that life began much earlier. We, together with
all complex multicellular organisms, belong to the eukaryotes and each
individual is the end product of 2.7 billion years of eukaryote evolution. To
appreciate the wonder of each group of animals and plants, we need to consider
their history, and how they came to be where they are. The history of life is
an interaction of biotic evolution with the complexities of the planet’s
geological history, continuous fluctuations in climate and vast spans of time,
punctuated by frequent local and rare global cataclysmic events.
Despite
the numerous perils facing marine environments, most extinctions in the current
episode have been confined to terrestrial and non-marine aquatic environments.
Many vertebrates are under intense threat, populations have crashed, some have
become extinct, others are close to extinction (Ceballos et al., 2015). This
justly generates much human anguish. However, over 99% of animals are
invertebrates (Tetley et al., 1999). Invertebrate extinctions are massive, most
notably in the biodiverse terrestrial faunas of tropical forest. Invertebrates
form the foundation on which ecosystems and many life forms are totally
reliant. If we are concerned about biodiversity loss then their story needs to
be told and their fate needs to be a focus of our attention.
Different
invertebrate groups can provide different perspectives. Numerous insects have
become extinct without the losses being recorded (Hochkirch, 2016). Insect
populations have crashed in many parts of the world, from Europe (Hallmann, et
al., 2017) to the tropics (Lister and Garcia, 2018), along with their
associated predators such as many reptiles, amphibians and birds. However, some
of these results are controversial (Willig et al., 2019; Lister and Garcia,
2019) and although chemical controls are the main suspects, notably
neonicotinoids, there is often no proven link to causes of declines in
abundance. Despite overwhelming subjective evidence for massive drops in insect
numbers (Vogel, 2017), we have a problem in that despite numerous recording
schemes of insect species occurrence, there have been few long-term studies of
insect species abundance.
Molluscs
can provide a different perspective. They are a major invertebrate group in
terms of both biodiversity and biomass (Bar-On et al., 2018), and land snails
can be particularly informative about patterns of diversity and current
extinction events (Lydeard et al., 2004). I am interested in and concerned
about the whole of living diversity but land snails have several attributes
that render them particularly informative about all scales of evolution and
changes in the environment, such as climate and habitat changes through time.
Good examples of this were made available when the channel tunnel was
excavated, giving access to previously hidden fossil-rich deposits (Kerney et
al., 1980; Preece and Bridgland, 1999), and examples of successive horizons are
equally informative in tropical ecosystems such as in Jamaica (Goodfriend and
Mitterer,1988, 1993; Paul and Donovan, 2005; Donovan et al., 2013).
Land
snails are not what is termed a ‘natural group’. In the distant past, several
aquatic and only distantly related snails colonised the land independently
(Little, 2009). Some such as the terrestrial Caenogastropoda are derived from
winkle-like ancestors, they have separate sexes and seal the apertures of their
shells with a plate that is attached to the top of their tails; they are
numerous in parts of the tropics, less so in temperate regions. The other main
groups included in the Pulmonata have more developed lungs and are
hermaphrodites, they occupy all habitat types in which land snails occur from
deserts to marshland, from leaf litter to the heights of tree canopies.
Snails
generally have relatively poor powers of dispersal but, given sufficient time,
a few are passively dispersed over long distances, by hurricanes for example.
There is also strong evidence of long-distance dispersal of snails by birds
(Gittenberger et al., 2006; Leeuwen et al., 2012). In the short to medium term,
most snails are confined to their location in ways that many other organisms
are not. Unlike the majority of terrestrial arthropods, they cannot run or fly;
the vulnerability of their delicate bodies is primarily offset by retracting
and taking refuge within their shells. This limited motility makes them
vulnerable to extinction when conditions change. However, where natural
habitats are continuous, they can successfully change their distributions,
including latitudinal and altitudinal changes, with the shifting of ecosystems
in response to climate change. The shells may sometimes be delicate but many
are robust and may survive long after the snail has died. In several lineages
the shells are vestigial or lost altogether. There is a continuous transition
between snails, semi-slugs and slugs but for convenience and to allow
generalisations to be made, slugs are not considered here.
Whatever
the season, a good measure of what snails are present in an extant habitat can
be gained by collecting their shells. Thus, natural history museums around the
world often hold extensive collections of shells that require no special
procedures for their preservation and storage. Where well documented, these
collections provide a partial record of where snail species were found in the
past. Day to day routine identifications and classifications may be carried out
solely by examination of snail shells. However, more sophisticated methods of
morphological study of internal organs and molecular methods are essential for
more critical studies. Such studies have shown that numerous cryptic species
and even higher taxonomic categories can be recognised compared to
identifications based solely on shell characters.
Extinctions on oceanic islands
and on continents
The
unique radiations of animal diversity that occurred on oceanic islands, most
less than 10 myr old, took place in habitats that were free of the
taxonomically diverse and highly evolved systems of predators and competitors
that had developed on continents through tens and hundreds of millions of
years. This contributed to island biotas’ vulnerability to human introductions
of continental species that had attuned to the harsh selective pressures from
which the evolution of oceanic island species had been sheltered. The arrival
of humankind on oceanic islands has progressively led to the widespread loss of
oceanic island species, their unique habitats and ecosystems (Fordham and
Brook, 2008). Recorded extinctions of land snails on oceanic islands exceed
those of all other groups combined (Lydeard et al., 2004).
Losses
on continental land masses through human activity also have a long history but
they have generally been less visible. We are now losing continental species at
an unprecedented rate, with complete and complex ecosystems that have evolved
over many millions of years. This is a growing tragedy of the Anthropocene.
Although these large-scale extinctions are now taking place on continental land
masses, the circumscribed nature of continental islands (fragments of
continental tectonic plates) allows them to be examined as discrete units and
used as exemplars for what is going on in continents as a whole. To this end,
aspects of the fauna of two of the world’s largest and very different tropical
islands, Madagascar and Sumatra, are considered here in the context of regional
faunas with particular reference to their land snails.
Sumatra
Sumatra
epitomises a manifestation of the sixth mass extinction and demonstrates the
disaster that is rapidly unfolding in southeast Asia (Sodi et al., 2004;
Hughes, 2017). What has happened in Sumatra has significantly influenced my
thinking on extinction because nearly all of the lowland and much of the
montane forest habitats, which previously blanketed the landscape, have been
lost in my lifetime (figure 1). At 443,066 square kilometres, an area greater
than twice the size of Great Britain, Sumatra is a large, geologically complex
island about 3.3 times the area of Peninsula Malaysia. It was repeatedly connected
to the continental land mass as an integral part of Sundaland, a southeast
Asian global biodiversity hotspot, throughout glacial episodes. Thus, during
the past 2.6 million years of ice ages, its biotic history and composition was
as a part of continental southeast Asia (Woodruff, 2010).
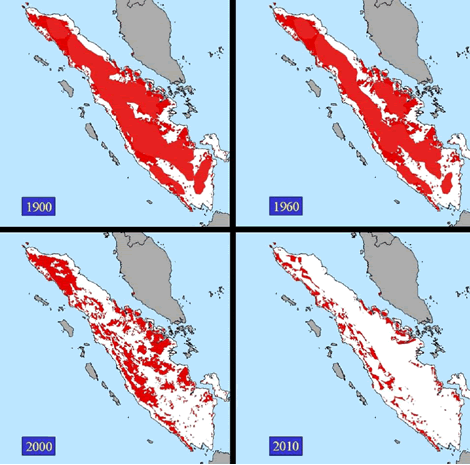
Figure 1. Forest loss on
Sumatra due to logging and conversion to agriculture. The red depicts remaining
forest cover. © WWF.
Straddling
the equator at an angle of about 45°, Sumatra is geologically a part of
continental Eurasia and part volcanic in origin, its southern border lies along
the subduction zone of Sundaland and the Indo-Australian plate and it is part of
one of the most tectonically active areas in the world. Frequent volcanism,
earthquakes and tsunami impact on the biota. Notably, the explosive eruption of
Mount Toba 73,500 years ago must have had a massive impact on southeast Asia
and peninsula India’s biota through ash deposition (Bühring and Sarnthien,
2000; Jones, 2007). Nevertheless, a mixture of plains and complex mountain
systems offered a diverse array of forest habitats in Sumatra providing it with
some of the richest biodiversity on the planet. Despite enormous expenditure on
conservation effort, lowland forest was close to being entirely lost at the end
of the twentieth century (Whitten, et al., 2001), just a few diminishing
patches remain. Iconic mammals such as the Sumatran tiger, rhinoceros, elephant
and orangutan are all widely recognised as being critically endangered.
Benthem
Jutting (1959) listed just 192 species of land snails from Sumatra and a few
have been described since (Maassen, 1999, 2000; Páll-Gergely, 2017). However,
we have little idea of how many species might have been present in Sumatra 60
years ago; it is likely to have been closer to 2,000 than 200. What is clear is
that with most natural habitat destroyed in Sumatra, many of the endemic
species will be extinct. The invertebrate diversity of Sumatra’s lowland
forests was never studied methodically and now never can be. This demonstrates
what scientists mean when they speak of species going extinct before they have
even been described. The loss of 98% of forests in large parts of Indonesia is
projected by 2022 (Hughes, 2017, 2018). Sumatra stands out because the scale of
destruction has been so rapid. It is not just forests that are disappearing.
Limestone hills are habitat islands rich in biotic diversity with particularly high
snail diversity and density. The more isolated a limestone hill, the greater
the likelihood that it possesses high levels of biotic endemism and the greater
the risk of its destruction for limestone extraction.
In
Sumatra the main driver of habitat loss and consequent extinctions was
explicitly and succinctly identified by Whitten et al. (2001), three pages of
essential reading for anyone who wants to understand where conservation efforts
in Sumatra stood at the turn of the century. What happened in Sumatra should
and could have been avoided, and at least mitigated, but it wasn’t. Despite
massive conservation effort, all of the management plans, political accords and
expenditure of unknown millions of US dollars, deforestation continued
unabated. Big business and political corruption, both equally ruthless, rode
over any conservation efforts. The whole purpose of the flourishing academic
field of conservation was questioned by Whitten et al. (2001, p.1):
In
these same three decades we have also seen conservation biology rise as a
respected and attractive discipline, with great successes in producing
journals, books, and students. But if conservation biology is ineffective in
helping to stop something as globally significant as the devastation of
Indonesian forests, then what, please, is the point of it?
Sumatra
has a human population of approximately 52 million, around 90.5 people per km²; the human population of Indonesia as
a whole has increased to 3.5 times its 1955 level. For comparison, consider Sri
Lanka, which has a population of just over 20 million, 340 per km², about twice
its 1955 level with 82% living in rural areas. Much forest has been lost in Sri
Lanka but it has a number of relatively well-protected areas and has so far
retained a rich biota including large mammals such as thriving populations of
elephants and leopards. It appears that local human population pressure in
Sumatra, with 6.75 times the area of Sri Lanka, might not have been the major
driver of habitat loss and extinctions. It is in fact clear that the primary
driving force of habitat loss and extinctions in Sumatra is external,
consumption of its resources around the world, an insatiable demand for its
products, notably palm oil and timber, facilitated by greed and corruption.
Madagascar
With
an area of 587,041 km², Madagascar is a large continental fragment of Gondwana,
one of the two great landmasses that separated from the single land mass of
Pangaea with the opening of the Tethys Ocean about 175 million years ago. The
southern continent of Gondwana was separated from the northern land mass of
Laurasia for about 100 myr. During the subsequent breakup of Gondwana,
Madagascar together with India, separated from Antarctica about 125 mya, having
separated from Africa some 20 myr earlier. Around 88 mya, India separated from
Madagascar. Madagascar moved slowly north to its current longitude whereas
India was drawn north much more rapidly until it collided with Eurasia (Smith
et al., 1994). India is still thrusting into Asia and continues to force up the
Himalaya.
The
world was a much warmer place throughout most of Madagascar’s existence and
large tracts of what is currently dry land were covered in shallow sea. The
limestone deposited during these marine incursions provided a particularly rich
habitat for limestone biotas including land snails. 88 myr of isolation have
endowed Madagascar with a truly unique biota. Unlike Sumatra, Madagascar is an
ancient land mass and geologically is relatively stable, although there is some
tectonic activity and it possesses dormant volcanos (Pratt et al., 2016). The
closest Indian coastline is now some 3,800km away but it was of course closer
throughout much of the past 88 myr and there were periods when a series of
islands, now largely submerged, provided potential stepping stones for biotic
transfer. Mainland Africa, currently some 450km away at its closest point, has
remained in relatively close proximity throughout.
Whereas
the climate in Sumatra is hot and wet throughout the year, the climate in Madagascar
is much more complex being dominated by the joint action of the moist southeast
trade winds and the wet northwest monsoon. The east coast has a high annual
rate of precipitation but on reaching the plateau prevailing winds have lost
much of their humidity resulting in only light rain and mist, leaving the west
in a rain shadow; areas of the southwest are semidesert. Madagascar’s biota has
exploited the diverse range of habitats that are strongly influenced by this
climate. For agriculture, the climatic variations across Madagascar present
challenges ranging from severe drought to deluge flooding.
A
large proportion of Madagascar’s biota is endemic but, during its 88 Ma of
isolation, rare dispersal events across the seas introduced new biotic elements
from further afield, some of which radiated into significant new components of
Madagascar’s biota. A classic example is the lemurs, now confined to
Madagascar. Molecular phylogenetic and anatomical evidence suggests that the
ancestor of the currently recognised 111 species and subspecies, 20% of the
world’s primate species, reached Madagascar from Africa at around 54 mya
(Martin, 2000; Mittermeier et al., 2008). Following the loss of natural
habitats (figure 2), some 95% of lemur species are on the threshold of
extinction. The IUCN Species Survival Commission (SSC) raised over US$8 million
to spearhead efforts to save them with a 3-year conservation plan in 2013
(IUNC, 2013). An IUCN updated assessment in 2018 (Bristol Zoo, 2018) showed
that, despite some local successes, the threat of lemur extinction has
increased.
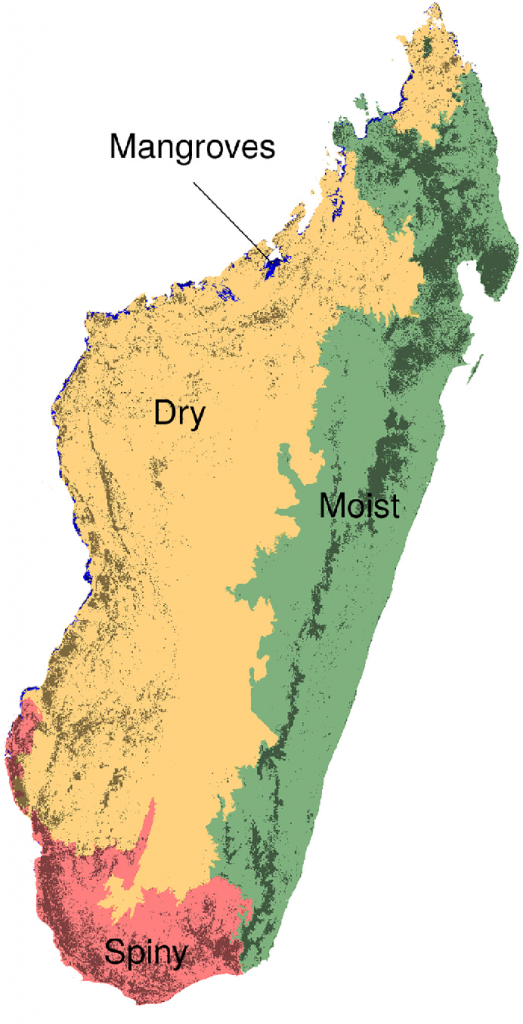
Figure 2. Ecoregions and forest
types in Madagascar. Madagascar can be divided into four climatic ecoregions
with four forest types: the moist forest in the East (green), the dry forest in
the West (orange), the spiny forest in the South (red), and the mangroves on
the West coast (blue). The dark areas represent the remaining natural forest
cover in 2014. Forest types are defined on the basis of their belonging to one
of the four ecoregions. (Reproduced from Vieilledent et al., 2018).
Evidence
strongly supports two Africa-to-Madagascar dispersal events for chameleons
across the Mozambique Channel, one at about 65 mya, the second at about 47 mya
(Tolley et al., 2013). These two rare events gave rise to the amazing
diversifications of chameleons in Madagascar, about half of the world’s
chameleon species diversity. According to an assessment by the SSC, 52% are
threatened, including 5 species that are critically threatened and 18% are near
threatened (Hance, 2014).
As
with Sumatra, by the middle of the twentieth century, some 200 species of land
snails had been recorded from Madagascar. However, following intensive studies,
notably by Emberton between 1990 and 2009, the total number reached about 1100
(Slapcinsky, 2014). Despite their commendable efforts, it is impossible for a
handful of people to have described most of the land snails of the 587,041 km²
of Madagascar. With no one dedicated to their study, there is unlikely to be
the same pace in new species descriptions. Many will now be extinct but there
may have been about 2,000 species in total.
Despite
its 88Ma history as an isolated land mass, Madagascar’s snail fauna has origins
that extend across all directions of the Indian Ocean. The most distinctive
components, the 115 described species of Acavidae, are considered to be
Gondwanan relicts (Emberton, 1999). Their ancestors were distributed across
Gondwana prior to its breakup and acavids are now found only on continental
fragments of Gondwana: South America, Africa, Madagascar, the Seychelles, Sri
Lanka and Australia. The mode of dispersal of acavids is to sit tight on
continents for tens of millions of years and wait for plate tectonics to do the
work for them. The acavids possess large, often brightly coloured shells and
produce disproportionally large, bird-like eggs. With even their hatchlings
being relatively large, their size seems likely to have contributed to the fact
that they appear not to have spread across oceans by natural means.
The
genera Kalidos, Boucardicus and Tropidophora have
radiated into numerous Madagascan species. There is evidence that the ancestor
of Kalidos made its way to Madagascar from southeast
Asia, possibly via India. Boucardicus shows similarities with genera found in
south and southeast Asia but, with similar looking fossils in 100 myr old
Burmese amber, it is clear that these groups have been around for a very long
time and their relationships need to be established by molecular methods A
different distribution pattern is shown by Tropidophora, which
also occurs on the opposite land area of Africa, on the Comoros and the
Seychelles. Related genera are found around the Indian Ocean from Socotra,
mainland Yemen and Oman, with a separate genus and two species occurring in the
Western Ghats, India (Raheem et al., 2014).
They
may be more ancient arrivals but the radiations into numerous species within
single genera such as Kalidos and Tropidophora are
suggestive of relatively recent arrivals of these genera into Madagascar,
possibly during the Miocene (23 mya to 5.3 mya).
What
the lemurs, chameleons and land snails have in common with much of Madagascar’s
and other tropical biotas is that most species have very restricted
distribution ranges within the complex mosaic of naturally diverse habitats.
The majority of Madagascar’s land snails have been described on the basis of a
few individuals from a single locality, some from partially weathered shells of
species that may have already been extinct at the time of their description.
Habitats cannot be transformed by human activity without the consequent
wholesale loss of localised species. The composition and diversity of land
snails conveys the long biotic history of Madagascar better than any vertebrate
group and their Anthropocene extinction is already well underway.
There
have been years of debate and a lack of consensus on the causes of tropical
diversity but, whatever the mechanism, high diversity dominated by limited
range distributions is widespread in the tropics and has been for millions of
years (Brown, 2013). Despite the age of this biotic diversity of lineages in
the wet tropics, they are now extremely vulnerable to habitat loss and
transformation because of their often-restricted distributions and their being
surrounded by a matrix of human transformed habitats.
With
well-established recognition of its incredibly rich biodiversity and extreme
levels of endemism, Madagascar has been a priority target of international
research and conservation effort for decades (National Research Council, 1980;
Myers, et al., 2000; Goodman and Benstead, 2005). Efforts reached a height
during the implementation of a series of National Environment Action Plans
between 1993 and 2008, when hundreds of millions of US$ were spent on over 500
environmentally-based projects. Eight Millennium Development Goals were
established for a fifteen-year period from 2000, supported by the Madagascar
Millennium Development Goals National Monitoring Survey (INSTAT, 2014) and the
protected areas network was expanded threefold. Projects aimed at sustainable
development and reducing poverty have failed, in fact none of the Millennium
Development Goals were met nor was progress made towards them, and relentless
deforestation continues unabated (Waeber et al., 2016; Vieilledent et al.,
2018). The protected area network is widely ignored.
Madagascar
is larger than Sumatra but has a smaller human population estimated at 20-27
million, approximately half that of Sumatra (population density of Madagascar
some 46 per km²; Sumatra 90.5 per km²). It might be thought that human
population levels would have less impact. However, Madagascar is in a sorry
state (UNIC, 2019):
The
country’s health and education systems are not really working, they are
crumbling; In the last two years 77 % of the population have been living on
less than 1.25 dollars a day.
More
than 92% of Malagasy live on less than US$ 2 a day (World Bank, 2013).
Madagascar’s infant mortality rate is over 5% and three-quarters of the
population live in rural areas. The estimated median age in 2017 was 18.7,
compared with 40.1 for the UK, indicating that population growth is hardwired
into the immediate future. Although the total fertility rate (TFR) has fallen
from 7.3 in 1960 to 4.18 in 2016, Madagascar’s TFR is still nearly double
replacement level. Logging and mining controls are ineffective. Large numbers
of people have little choice other than to take what they can from their
environment, regardless of any conservation needs. Traditional slash-and burn
agriculture is increasingly practiced in desperation and on a completely
unsustainable scale, destroying natural habitats. They are not alone. As
pointed out in the executive summary of the World Conservation Strategy
(IUCN-UNEP-WWF, 1980, p. vi):
…
hundreds of millions of rural people in developing countries, including 500
million malnourished and 800 million destitute, are compelled to destroy the
resources necessary to free them from starvation and poverty.
Reponses to the biodiversity
crisis
“It
is far better to grasp the Universe as it really is than to persist in
delusion, however satisfying and reassuring.” – Carl Sagan
The
cases of Sumatra and Madagascar demonstrate both the scale and causes of
biodiversity loss. In Sumatra conservation efforts have failed in the face of
insatiable global demand for its resources along with greed and corruption,
while in Madagascar endogenous factors, including poverty and population
growth, have been the most significant causes of habitat destruction. Given the
scale of biodiversity loss as exemplified by these islands, the following
sections go on to consider some aspects of the global responses by governments,
conservation agencies and academics.
Earth
Optimism was launched in 2017 with a series of meetings including in Washington
(Smithsonian Conservation Commons, 2017), in Cambridge (Cambridge Independent,
2017) and London (ZSL Institute of Zoology, 2017). The momentum of Earth
Optimism continues and a Conservation Optimism summit was held at Oxford in
2019 (University of Oxford, 2019).
A
number of justifications for Earth Optimism have been put forward. One
suggestion is that such an approach is essential in order to engage with the
public. Others suggest that people who are seeking careers in the field need to
be encouraged by a sense of optimism and that it is needed to secure corporate
and government funding. To quote from the ZSL Institute of Zoology (2017):
Budding
and perennial conservationists need to feel inspired and continue in the
profession, not put off by pessimism. The public, businesses and government
need to know that their actions can make a difference.
However,
promoting optimism in this way exaggerates successes in relation to the size of
the problem and ultimately is not only inappropriate but misleading.
Importantly, it infantilises the public by assuming that they will only engage
with optimistic information and runs the risk of undermining trust in
scientific integrity. Perhaps the most worrying aspect of Earth Optimism is
that in focussing on the celebration of those success stories the overriding
issues of human overpopulation and overconsumption that are driving mass
extinction are ignored.
The
Convention on Biological Diversity (CBD) grew around the concept enshrined in
Article 1 of the Convention (CBD, 1992, p.3):
The
objectives of this Convention, to be pursued in accordance with its relevant
provisions, are the conservation of
biological diversity, the sustainable use of its components and the fair and
equitable sharing of the benefits arising out of the utilization of genetic
resources, including by appropriate access to genetic resources and by
appropriate transfer of relevant technologies, taking into account all rights
over those resources and to technologies, and by appropriate funding.
With
almost universal celebration and after years of preparation, the CBD was
launched in Rio de Janeiro in 1992. Bureaucracies proliferated and numerous
agencies were created so that many thousands are employed at great cost in
developing both national and international plans and in attending massive
international conferences. From a brief initial focus on conservation it soon
became a behemoth of international agencies seeking to extract funding
resources for development, programmes that had little if anything to do with
biological conservation. It is an empire of vested interests that has failed to
deliver conservation objectives. Extinctions continue unabated (Anon, 2016) and
bio-nationalism has impeded international conservation efforts. The United
Kingdom’s flagship CBD programme, the Darwin Initiative, epitomises the change
in direction that effectively constitutes a high-jacking of the CBD agenda from
a biodiversity capacity building focus to a development agency based on poverty
alleviation. Worthy as these objectives may be in their own right, they have
not even slowed the current scale of biodiversity loss.
Brown
(2015, p.1) provided an impeccable and succinct demolition of the notion of
sustainable development:
Unfortunately,
“sustainable development,” as advocated by most natural, social, and
environmental scientists, is an oxymoron. Continual population growth and
economic development on a finite Earth are biophysically impossible. They
violate the laws of physics, especially thermodynamics, and the fundamental
principles of biology. Population growth requires the increased consumption of
food, water, and other essentials for human life. Economic development requires
the increased use of energy and material resources to provide goods, services,
and information technology.
Sustainable
development goals can provide neither sustainability nor a pathway to halting
the sixth mass extinction. However, governments, numerous agencies and
commercial enterprises around the world, together with academics, fail to
acknowledge their flawed nature. For example, the UN Sustainable Development
Goal 15, life on land (UN, 2019), should be of key importance to biodiversity
loss. Goal 15 seeks to sustainably manage forests, combat desertification, halt
and reverse land degradation and halt biodiversity loss. However, there are no
realistic mechanisms or new ideas put forward of how this could be achieved on
a scale commensurate with the problem. Reference is made to the Lion’s Share
Fund, a worthy programme but one that can only have a tiny, if useful, impact
on biodiversity loss.
A
wide range of conservation activities are pursued by the IUCN including the
formulation and development of international agreements such as the 1974
Convention on International Trade in Endangered Species, and the CBD. Together
with partner organisations the IUCN is pursuing a pathway to conservation based
on the concept of sustainable development. However, their Red Listing system
(IUCN, 2019) is unique in aiming to provide hard data of extinction risk in
support of conservation and, particularly for large vertebrates, has many
merits. An example of an outstanding achievement with invertebrates is the IUCN
Red List of European Terrestrial Snails (Neubert et al., 2019), which was
developed from many years of recording schemes and input from numerous
contributors. However, the situation for a single species, the world’s largest
cat, the tiger, is illustrative of the problematic nature of the IUCN’s
approach. Project tiger (National Tiger Conservation Authority, 2019) has been
running for nearly 50 years, has cost millions of US$, involved thousands of
people and supported numerous careers. Yet controversy surrounds the results of
surveys and in obtaining accurate figures of tiger numbers (Karanth, 1995;
Karanth et al., 2017; Mazoomdar, 2019). In contrast, only a handful of people
have been dedicated to surveying land snails in the tropics, a totally
inadequate number for assessing the status of numerous often tiny snails in the
world’s rainforests. For most species and areas, it is not remotely possible to
obtain accurate information within a timeframe commensurate with the urgency
imposed by the rate of habitat loss and extinctions. We remain in ignorance or,
in Red List terminology, data deficient. The WWF sets out its agenda in the Living Planet Report 2018: Aiming higher.
This would be a highly commendable document but for the fact that it ignores
the major underlying causes of the problems it identifies: human overpopulation
and the ecologically impossible concept of sustainable development. Together
with overpopulation, economics is at the heart of our current unsustainable
trajectory. Global economics is currently based on growth and benefits from
population growth and increased wealth with consequent increases in consumption.
Clearly, this is not to suggest that reduction in poverty is in itself
undesirable but that it has inevitable, undesirable and unsustainable
consequences. Much can be done to mitigate but not remove the impact of
increased consumption, for example, by the reduction and ultimate elimination
of the use of fossil fuels and by modifications to diets. However, the human
ingenuity argument fails to recognise that improvements that science and
technology have brought to human welfare have not been shared with the natural
world. While economic growth is necessary to improve the welfare of the world’s
poor, endless economic growth to satisfy the wants of an ever-increasing global
consumer class is simply unsustainable.
The
desperately urgent need for a strategy aimed at establishing an inventory of
what remains of living diversity has been recognised for some considerable time
(Wheeler, 1995). It is utterly shameful that this has not happened. The Earth
Biogenome Project (2019) is wildly overambitious to the extent of being utterly
unrealistic in aiming to sequence, catalogue and characterize the genomes of
all of Earth’s eukaryotic biodiversity over a period of ten years. This to
include what it estimates as the 80-90% of eukaryotes that have yet to be described.
Over two centuries of just searching out living diversity has left us with a
long way to go and locating the whole range of species is a long way off, even
though that unknown number is rapidly declining. The Earth Biogenome Project
(EBP) is described as a ‘moonshot for biology’ (EBP, 2019). It might have
launched but it cannot reach its ten-year scheduled destination. Despite its
extravagant claims as a means of contributing to the conservation of species
(Lewin, et al. 2018), as it stands, it will not do so. Sequencing eukaryote
diversity might provide employment for numerous scientists, if only for a
decade; it can satisfy human curiosity and yield new means of exploiting
natural resources but it will not contribute to preventing the loss of natural
habitats or reduce human driven climate change. Their analogy with space
exploration seeks to link the EBP with human achievements that are widely
celebrated and have numerous indirect benefits. However, all such scientific
endeavours should be judged by the proportionality of effort and cost in the
context of priorities on our own planet and the destruction we are collectively
inflicting on it. In the context of the sixth mass extinction, the
disappearance of its subject matter, and unless balanced in new directions, the
EPB objectives are a self-satisfying indulgence. This is analogous to a
consortium of hospitals of global prestige around the world being obsessed with
gaining academic stature while ignoring countless thousands of dying patients.
Curiosity
driven research provides inspiration and motivation for learning about the
universe and the EPB has the merit of recognising the scale of the issue and
timeliness, if not the constraints. In addition, the EPB fails to accept the
requirement for voucher collections that are needed to support the molecular
sequencing. Unless intended as an abstract exercise, or an exclusively
molecular based alternative to existing concepts, it is meaningless to sequence
samples without being able to relate them to physical entities. Voucher
specimens are the preserved samples linked to the genomes to be sequenced. Some
species might be sufficiently well known for their identity to be accepted but
such are insignificant compared to the vast majority of described but poorly understood
species and for undescribed species. Traditionally, voucher samples were whole
preserved specimens and there is still a place for these but detailed images
could in many instances be a practical option in combination with tissue
sampling. In addition to traditional methods of preservation and frozen tissue
collections, it is possible to prepare specimens in an ultimate state of
preservation by preserving viable cells, cell lines, without sacrificing or
harming the animal. Most importantly such preservation can underwrite all
traditional conservation efforts. If this were included in the EBP protocols
then it would completely transform the value of the programme. This is the
obvious direction in which resources and research efforts should be directed.
It is early days and there are numerous difficulties in extending the practice
to a wide range of species but cryogenically stored viable sperm and egg cells
are already being used as a measure to conserve species close to extinction
(Hermes et al., 2018) and viable somatic cells can potentially be cloned. Thus,
it is not only possible to conserve and utilise genetic diversity of threatened
species but preservation of viable cells offers the potential to restore
species if they should become extinct (Naggs, 2017), together with associated
organisms such as their gut biota. Viable cells of extinct species are already
being stored. The Hawaiian tree snail Achatinella apexfulva, supposedly the first recorded extinction
of 2019, was given extensive media coverage including by the National
Geographic (Wilcox, 2019) and the Natural History Museum (Pavid, 2019). Living
cells of Achatinella apexfulva are cryogenically stored in the San Diego
Zoo Institute for Conservation Research’s (2019) Frozen Zoo. As with Kew’s
Millennium Seed Bank, this material could potentially be cryogenically stored
for hundreds of years and should be the routine mode of preservation. Who can
say what future capabilities might be but, if we do not act now, whatever
current and future potential value they might have will be lost forever and
options for their use will not exist.
One
hurdle to surmount is that access to specimens has become much more difficult
and complicated. The way forward is to establish and nurture long-term
relationships, particularly with biodiverse countries. The pilot project that I
ran in 2013 demonstrated the value of collaboration and that viable cell
preparation could be routinely added to existing field practice (Naggs, 2017).
The sixth mass extinction should position natural history in the forefront of
scientific endeavour to record and conserve living diversity in an urgent
structured, focussed and relevant way.
The
Intergovernmental Panel on Climate Change (IPCC) is the United Nations body
invested with the task of evaluating the science related to climate change.
Scientific evidence is not determined by consensus but the overwhelming
assessment of scientific information is clear and cannot be ignored, humankind
is causing global warming. Where the IPCC has failed is in recognising that by
our very existence and ever-growing numbers, we cannot avoid global warming.
Indeed, there is clear evidence that the rise in CO2 and CH4 began
some 7,000 years ago with human driven deforestation and the development of
agriculture and livestock tending (Ruddiman, 2014, and references therein).
Furthermore, Ruddiman presents the case that without anthropogenic influence,
we would already have entered a new ice age. We undoubtedly need to take steps
to mitigate global warming but, even when we deal with eliminating fossil fuel,
as we must, the transformed landscapes and biomass of humans and their
livestock will continue to deliver elevated CO2 and CH4 above natural levels.
Climate
change is integral to earth history and happens regardless of human activity,
sometimes very rapidly. It is instructive to recognise that throughout much of
earth’s history CO2 levels have been much higher
and global temperatures have been much higher than they are now and natural
events could overwhelm any anthropogenic changes. We should be prepared for the
inevitability of climate change in one direction or another. Living diversity
has accommodated to climate change throughout its existence. What is unique
about the current situation is that natural forest landscapes have been
transformed into a mosaic of modified (largely agricultural) habitats and
fragmented natural forest, the forest remaining as isolated and shrinking patches.
Combined with climate change, the barriers to dispersal will precipitate a new
catastrophic wave of extinctions and there is an urgent need to provide habitat
corridors and to be prepared to intervene with the seeding of new habitats that
develop in response to climate change.
Taking stock
The
history of life on earth shows it to be a dynamic mix and match of blending and
separating of biotas through time. In addition to the many other human impacts
on the natural world is an acceleration of this mixing to a global scale and
breakdown of geographical isolation. In the mixing of biotas there are a few
winners and numerous losers. Increased mixing leads to a reduction in local
endemism and thus a reduction in biodiversity.
Sumatra
and Madagascar demonstrate that both local and global human population
pressures produce the same outcome, habitat loss and extinction. Socioeconomic
factors and human numbers present an unsolvable conundrum. There is a
widespread belief that human ingenuity can solve such problems. Proponents of
this view correctly point out that living standards throughout much of the
world have improved dramatically through the application of science and
technology. The same cannot be said of natural environments that have suffered
as a consequence. We are already a long way down the road of destroying the
natural world. Habitat fragmentation combined with climate change will
precipitate a surge of extinctions in the near future. Conservation is thriving
as an academic discipline and can point to success stories but overall it is a
failure. Such an assessment is often dismissed as a doom and gloom scenario but
there are many opportunities to act in positive ways. There are too few
habitats approaching pristine condition for them to be the sole focus of
conservation effort and some transformed habitats retain significant subsets of
biological diversity and need to be integrated into conservation practice.
Again, snails show the way in demonstrating that some forest fragments and
transformed habitats can still support a significant subset of forest species
(Raheem et al., 2008, 2009; Triantis et al., 2008), although such transformed
habitats are being rapidly lost to more intensive modes of agriculture.
We
have to accept that we cannot halt large scale extinctions and act accordingly.
A new drive for a zoological species inventory, that also conserves
biodiversity and secures options for the future, is essential in the context of
massive species loss. For conservation in the here and now, new and direct
emergency action is needed to protect natural habitats. One overriding need is
for a simple and straightforward mechanism for providing significant funding
for poor but biodiversity rich countries to protect natural habitats. Used for
the benefit of their human populations, this is possibly the only way to arrest
immediate biodiversity loss where it is driven by poverty. This is happening in
a small way but it needs to be on a huge scale, something appropriate for
private agencies and governments to engage with through the United Nations.
References
Anon,
2016. Biodiversity betrayed as nations fail miserably on conservation. New Scientist, [online]
8 December. Available at: <https://www.newscientist.com/article/mg23231034-300-biodiversity-betrayed-as-nations-fail-miserably-on-conservation/>
[Accessed 18 October 2019].
Bar-On,
Y.M., Phillips, R., and Miloa, R., 2018. The biomass distribution on Earth. Proceedings of the National Academy of Sciences. [e-journal] 115(25),doi/10.1073/pnas.1711842115.
Benthem
Jutting, Van S.S.W., 1959. Catalogue of the non-marine Mollusca of Sumatra and
of its satellite islands. Beaufortia,
7(83), pp.41-191, plates 1-2, map.
Bristol
Zoo, 2018. 95 percent of world’s lemur
species on edge of extinction. [online] Available at: <https://www.bristolzoo.org.uk/latest-zoo-news/lemur-species-on-edge-of-extinction>
[Accessed 12 October 2019].
Brown,
J.H., 2013. Why are there so many species in the tropics? Journal of Biogeography, 41(1), pp.8-22.
Brown,
J.H., 2015. The oxymoron of sustainable development. BioScience, [e-journal] 65(10), pp.1027–1029. https://doi.org/10.1093/biosci/biv117.
Bühring,
C., Sarnthien, M., 2000. Toba ash layers in the South China Sea: evidence of
contrasting wind directions during eruption ca. 74ka. Geology,28, pp.275-278.
Cambridge
Independent, 2017. Earth Optimism Day in Cambridge
to celebrate conservation success. [online] Available at: <https://www.cambridgeindependent.co.uk/business/earth-optimism-day-in-cambridge-to-celebrate-conservation-success-9051346/>
[Accessed 18 October 2019].
CBD,
1992. Convention on biological
diversity. [pdf] United Nations. Available at: <https://www.cbd.int/doc/legal/cbd-en.pdf> [Accessed
18 October 2019].
Ceballos,
G., Ehrlich, P.R., Barnosky, A.D., García, A., Pringle, R.M., and Palmer, T.M.,
2015. Accelerated modern human–induced species losses: entering the sixth mass
extinction. [pdf] Science Advances, 1(5), Available at: <https://advances.sciencemag.org/content/1/5/e1400253/tab-pdf>
[Accessed 18 October 2019].
Donovan,
S.K., Baalbergen, E., Ouwendijki, M., Paul, C.R.C. and van den Hoek Ostende,
L.W., 2013. Review and prospectus of the Late Pleistocene fauna of the Red
Hills Road Cave. Jamaica Cave and Kast Science,
40(2), pp.79-86.
Earth
Biogenome Project, 2019. Sequencing life for the future
of life. [online]
Available at: <https://www.earthbiogenome.org/> [Accessed 18 October
2019].
Emberton,
K.C., 1999. New acavid snails from Madagascar. American Malacological Bulletin, 15(1), pp.83-96.
Fordham,
D.A., Brook, B.W., 2008. Why tropical island endemics are acutely susceptible
to global change. Biodiversity and Conservation, 19,
pp.329-342.
Gittenberger,
E., Groenenberg, D.S.J., Kokshoorn, B., and Preece, R.C., 2006. Molecular
trails from hitch-hiking snails. Nature, 439, p.409.
Goodfriend,
G.A., and Mitterer, R.M., 1988. Late Quaternary land snails from the north
coast of Jamaica: local extinctions and climatic change. Palaeogeography, Palaeoclimatology, Palaeoecology,
63(4), pp.293-311.
Goodfriend,
G.A., and Mitterer, R.M., 1993. A 45,000-yr record of a tropical lowland biota:
the land snail fauna from cave sediments at Coco Ree, Jamaica. Geoscience Bulletin, 105(1), pp.18-29.
Goodman,
S.M., and Benstead, J.P., 2005. Updated estimates of biotic diversity and
endemism for Madagascar. Oryx, [e-journal] 39, pp.73-77. https://doi.org/10.1017/S003060530500012.
Hallmann,
C.A., Sorg, M., Jongejans, E., Siepel, H., Hofland, N, Schwan, H. and Stenmans,
W., 2017. More than 75 percent decline over 27 years in total flying insect
biomass in protected areas. PLOS ONE, [e-journal] 12(10): e0185809,
https://doi.org/10.1371/journal.pone.0185809
Hance,
J., 2014. Chameleon crisis: extinction threatens 36% of world’s chameleons. Mongabay. [online]
24 November. Available at: <https://news.mongabay.com/2014/11/chameleon-crisis-extinction-threatens-36-of-worlds-chameleons/>
[Accessed 12 October 2019].
Hermes,
R., Hildebrandt, T.B., Göritz, F. 2018. Cryopreservation in rhinoceros—setting
a new benchmark for sperm cryosurvival. PLOS ONE, [e-journal]
13(7), e0200154, https://doi.org/10.1371/journal.pone.0200154.
Hochkirch,
A., 2016. The insect crisis we can’t ignore. Nature, 539 p. 141.
Hughes,
A.C., 2017. Understanding the drivers of Southeast Asian biodiversity loss. Ecosphere, 8(1), Article e01624: [pdf] Available
at:
<https://esajournals.onlinelibrary.wiley.com/doi/pdf/10.1002/ecs2.1624>
[Accessed 12 October 2019].
Hughes,
A.C., 2018. Have Indo-Malaysian forests reached the end of the road? Biological Conservation, 223, pp.129-137, [online] Available at: <https://www.sciencedirect.com/science/article/pii/S0006320717320426>
[Accessed 12 October 2019].
INSTAT,
2014. Madagascar Millennium
Development Goals national monitoring survey. [pdf] Available at: <https://madagascar.unfpa.org/sites/default/files/pub-pdf/OMD_Summary_0.pdf>
[Accessed 12 October 2019].
IUNC,
2013. Lemurs of Madagascar three-year
conservation plan launched.[online] Available at: <https://www.iucn.org/content/lemurs-madagascar-three-year-conservation-plan-launched>
[Accessed 12 October 2019].
IUCN,
2019. The IUCN red list of threatened
species. [online]
Available at: <https://www.iucnredlist.org/> [Accessed 18 October 2019].
IUCN-UNEP-WWF,
1980. World conservation strategy.
[pdf] Available at: <https://portals.iucn.org/library/sites/library/files/documents/WCS-004.pdf>.
[Accessed 18 October 2019].
Jones
S.C., 2007. The Toba supervolcanic eruption: tephra-fall deposits in India and
paleoanthropological implications. In: Petraglia M.D., Allchin B. eds. The evolution and history of human populations in South Asia.
Dordrecht: Springer.
Karanth,
K.U., 1995. Estimating tiger Panthera tigris populations from camera-trap data
using capture—recapture models. Biological Conservation, 71(3),
pp.333-338.
Karanth
K.U., Nichols, J.D., Goodrich, J.M., Reddy, G.V., Mathur, V.B., Wibisona, H.T.,
Sunarto, S., Pattanavibool, A., and Gumal, M.Y., 2017. Role of monitoring in
global tiger conservation. In: Karanth K., Nichols J., eds. 2017. Methods for monitoring tiger and prey populations.
Singapore: Springer.
Kerney,
M.P., Preece, R.C., and Turner, C. 1980. Molluscan and plant biostratigraphy of
some late Devensian and Flandrian deposits in Kent. Philosophical Transactions of the Royal Society of London,
Series B, Biological Sciences, 291(1044), pp.1-43.
Leeuwen,
van C.H.A., Velde, van de G., Lith, van B., Klaassen. M. 2012. Experimental
quantification of long distance dispersal potential of aquatic snails in the
gut of migratory birds. PLOS ONE. [e-journal] 7(3): e32292. https://doi.org/10.1371/journal.pone.0032292.
Lewin,
H.A. et al., 2018. Earth BioGenome Project: Sequencing life for the future of
life. [pdf] Proceedings of the National
Academy of Sciences, 115 (17), pp.4325-4333. Available at: <https://www.pnas.org/content/pnas/115/17/4325.full.pdf>
[Accessed 18 October 2019].
Lister,
B.C., and Garcia, A., 2018. Climate-driven declines in arthropod abundance
restructure a rainforest food web. Proceedings of the National
Academy of Sciences, [e-journal]
115(44), e10397–e10406. https://doi.org/10.1073/pnas.1722477115.
Lister,
B.C., and Garcia, A., 2019. Reply to Willig et al.: Long-term population trends
in the Luquillo rainforest. Proceedings of the National
Academy of Sciences, [e-journal]
116(25), e12145-12146. https://doi.org/10.1073/pnas.1904582116.
Little,
C., 2009. The colonisation of land. origins and adaptations of terrestrial animals.
Cambridge: Cambridge University Press.
Lydeard,
C., et al., 2004. The global decline of nonmarine mollusks. BioScience, [e-journal] 54(4), pp.321–330, https://doi.org/10.1641/0006-3568(2004)054[0321:TGDONM]2.0.CO;2.
Martin,
R.D., 2000. Origins, diversity and relationships of lemurs. International Journal of Primatology, [e-journal] 21(6), pp.1021–1049. https://doi.org/10.1023/A:1005563113546.
Maassen,
W.J.M., 1999. Some new records of Streptaxidae and Hypselostomatidae from
Sumatra, Indonesia, with descriptions of three new species. Basteria, 63(4/6), pp.121-126, [online] Available
at: <http://natuurtijdschriften.nl/download?type=document&docid=597163>
[Accessed 3 October 2019].
Maassen,
W.J.M., 2000. Notes on terrestrial molluscs of Sumatra, Indonesia, with
descriptions of ten new species (Gastropoda, Prosobranchia & Pulmonata). Basteria, 64, pp.137-150.
Mazoomdar,
J., 2019. Tiger count up but official photos show one in seven could just be a
paper tiger. The Indian Express, [online] 20 September. Available at:
<https://indianexpress.com/article/india/tiger-count-up-but-official-photos-show-one-in-seven-could-just-be-a-paper-tiger-6011560/>
[Accessed 3 October 2019].
Mittermeier,
R.A., Ganzhorn, J.U., Konstant, W.R. et al., 2008. Lemur diversity in
Madagascar. International Journal of
Primatology, [e-journal]
29, pp.1607-1656, https://doi.org/10.1007/s10764-008-9317-y.
Myers,
N., Mittermeier, C.G., da Fonseca, G.A., and Kent, J., 2000. Biodiversity
hotspots for conservation priorities. Nature, 403, pp.853-858.
Naggs,
F. Raheem, D., Platts, E., 2004. Some observations on Sri Lankan land snails
including the impact of the Indian Ocean tsunami on lowland snail faunas and
its importance as a major fossilisation event. The Malacologist, 45, [online] Available at: <http://malacsoc.org.uk/the_Malacologist/BULL45/tsunami.htm>
[Accessed 27 October 2019]
Naggs,
F., 2017. Saving living diversity in the face of the unstoppable 6th mass
extinction: a call for urgent international action. The Journal of Population and Sustainability, 1
(2), pp.67-81.
National
Tiger Conservation Authority, 2019. Project tiger, latest news.[online]
Available at: <https://projecttiger.nic.in/> [Accessed 18 October 2019].
National
Research Council, 1980. Research Priorities in tropical
biology. [e-book]
Washington, DC: The National Academies Press. https://doi.org/10.17226/19773.
Neubert,
E., et al., 2019. European Red List of terrestrial
molluscs: snails, slugs and semi-slugs. [pdf] Cambridge and
Brussels: IUCN. Available at: <https://ec.europa.eu/environment/nature/conservation/species/redlist/downloads/European_molluscs.pdf>
[Accessed 18 October 2019].
Páll-Gergely,
B., 2017. A new species of Alycaeidae, Pincerna yanseni n. sp. from Sumatra,
with the resurrection of the genus Pincerna Preston, 1907 (Gastropoda: Cyclophoroidea).
[pdf] Raffles Bulletin of Zoology, 65,
pp.213–219. Available at: <https://lkcnhm.nus.edu.sg/app/uploads/2017/04/65rbz213-219.pdf>
[Accessed 18 October 2019].
Paul,
C.R.C., and Donovan, S.K., 2005. Quaternary and recent land snails (Mollusca:
Gastropoda) from Red Hills Road Cave, Jamaica. Bulletin of the Mizunami Fossil Museum, 32,
pp.109–144.
Pavid,
K., 2019. Death of George the tree snail
marked the first extinction of 2019. [online] London: Natural
History Museum. Available at: <https://www.nhm.ac.uk/discover/news/2019/january/death-of-george-the-tree-snail-marked-first-extinction-2019.html>.
[Accessed 18 October 2019].
Pratt,
M.J., Wysession, M.E., Aleqabi, G., Wiens, D.A., Nyblade, A.A., Shore, P.,
Rambolamanana, G., Andriampenomanana, F., Rakotondraibe, T., Tucker, R.D.,
Barruol, G. and Rindraharisaona, E., 2016. Shear velocity structure of the
crust and upper mantle of Madagascar derived from surface wave tomography. Earth and Planetary Science Letters,
[e-journal] doi: 10.1016/j.epsl.2016.10.041.
Preece,
R.C., and Bridgland, D,R., 1999. Holywell Coombe, Folkestone: A 13,000 year
history of an English chalkland valley. Quaternary Science Reviews, 18, pp.1075-1125.
Raheem,
D.C., Naggs, F., Preece, R.C., Mapatuna, Y., Kariyawasam, L., and Eggleton, P.,
2008. Structure and conservation of Sri Lankan land-snail assemblages in
fragmented lowland rainforest and village home gardens. Journal of Applied Ecology, 45, pp.1019-1028.
Raheem,
D.C., Naggs, F., Chimonides, P.D.J., Preece, R.C., and Eggleton, P., 2009.
Fragmentation and pre-existing species turnover determine land-snail
assemblages of tropical rain forest. Journal of Biogeography, 36, pp.1923–1938.
Raheem,
D,C., Taylor, H., Preece, R.C., Aravind, N.A., and Naggs, F., 2014. A systematic revision of the land snails of the Western Ghats of
India. Bangkok:
Chulalongkorn University Press.
Ruddiman,
W.F., 2014. Earth transformed. New
York, USA: Freeman.
San
Diego Zoo Institute for Conservation Research, 2019. Frozen Zoo.[online] Available at: <https://institute.sandiegozoo.org/resources/frozen-zoo%C2%AE>
[Accessed 18 October 2019].
Slapcinsky,
J., 2014. Hypervariable or hyperdiverse, an independent assessment of the
taxonomically confusing land snail genus Tropidophora (Pomatiidae:
Littorinoidea: Caenogastropoda) in Madagascar. American Malacological Bulletin, 32(2), pp.259-266.
Smith,
A.G., Smith, D.G, and Funnell, B.M., 1994. Atlas of Mesozoic and Cenozoic coastlines.
Cambridge, UK: Cambridge University Press.
Smithsonian
Conservation Commons, 2017. Earth optimism summit 2017.[online]
Available at: < https://earthoptimism.si.edu/2017/> [Accessed 18 October
2019].
Tetley,
W., Ponder, W., and Lunney, D. eds., 1999. The other 99%: the conservation and biodiversity of invertebrates. Australia: Royal Zoological Society of
New South Wales.
Tolley,
K.A., Townsend, T.M., Vences, M., 2013. Large-scale phylogeny of chameleons
suggests African origins and Eocene diversification. Proceedings of the Royal Society, [e-journal] B 280, e20130184. http://dx.doi.org/10.1098/rspb.2013.0184.
Triantis,
K.A., Parmakelis, A., and Cameron, R.A.D., 2009. Understanding fragmentation:
snails show the way. Journal of Biogeography, 36,
pp.2021–2022.
UN,
2019. Sustainable development goals:
15, life on land. [online]
Available at: <https://www.un.org/sustainabledevelopment/biodiversity/>.
[Accessed 18 October 2019].
UNIC,
2019. Madagascar – the untold crisis. [online] Available at: <https://www.unric.org/en/madagascar/27072-madagascar-the-untold-crisis>.
[Accessed 18 October 2019].
University
of Oxford, 2019. Conservation optimism summit,
2019. [pdf] Available at: <https://summit.conservationoptimism.org/wp-content/uploads/2019/08/CO-Summit-Programme.pdf>
[Accessed 18 October 2019].
Vieilledent,
G., Grinand, C. Rakotomalala, F.A., Ranaaivosoa, R., Rakotoarijaona, J-R.,
Allnut, T.F. and Achard, F., 2018. Combining global tree cover loss data with
historical national forest cover maps to look at six decades of deforestation
and forest fragmentation in Madagascar. Biological Conservation, 222, pp.189-197. [online]
Available at: <https://www.sciencedirect.com/science/article/pii/S0006320718301125>
[Accessed 18 October 2019].
Vogel,
G., 2017. Where have all the insects gone? Science, 356(6338),
pp.576-579.
Waeber,
P.O., Wilmé, L., Mercier, J-R., Camara, C., Lowry, P.P., 2016. How effective
have thirty years of internationally driven conservation and development
efforts been in Madagascar? PLOS ONE,
[e-journal] 11(8), e0161115. https://doi.org/10.1371/journal.pone.0161115.
Wheeler,
Q.D., 1995. Systematics, the scientific basis for inventories of biodiversity. Biodiversity and Conservation, 4, pp.476-489.
Whitten,
T., Holmes, D., and MacKinnin, K.M., 2001. Conservation biology: a displacement
behaviour for academia. Conservation Biology, 15(1), pp.1-3.
Wilcox,
C., 2019. Lonely George the tree snail dies, and a species goes extinct.
National Geographic, [online]
8 January. Available at: <https://www.nationalgeographic.com/animals/2019/01/george-the-lonely-snail-dies-in-hawaii-extinction/>
[Accessed 18 October 2019].
Willig,
M.R., Woolbright, L., Presley, S.J., Schowalter, T.D., Waide, R.B., Heartsill
Scalley, T. and Woodruff, D.S., 2010. Biogeography and conservation in
Southeast Asia: how 2.7 million years of repeated environmental fluctuations
affect today’s patterns and the future of the remaining refugial-phase
biodiversity. Biodiversity and Conservation,
19(4), pp.919-941.
World
Bank, 2013. Madagascar: measuring the
impact of the political crisis. Washington, DC: World Bank.
[online] available at <www.worldbank.org/en/news/feature/2013/06/05/madagascar-measuring-the-impact-of-the-political-crisis>
[Accessed 18 October 2019].
Zimmerman,
J.K., González, G., and Lugo, A.E., 2019. Populations are not declining and
food webs are not collapsing at the Luquillo Experimental Forest. Proceedings of the National Academy of Sciences, [e-journal] 116 (25), 12143-12144.
https://doi.org/10.1073/pnas.1820456116.
ZSL
Institute of Zoology, 2017. Conservation optimism summit.
[online] Available at: <https://www.zsl.org/science/whats-on/conservation-optimism-summit>.
[Accessed 18 October 2019].